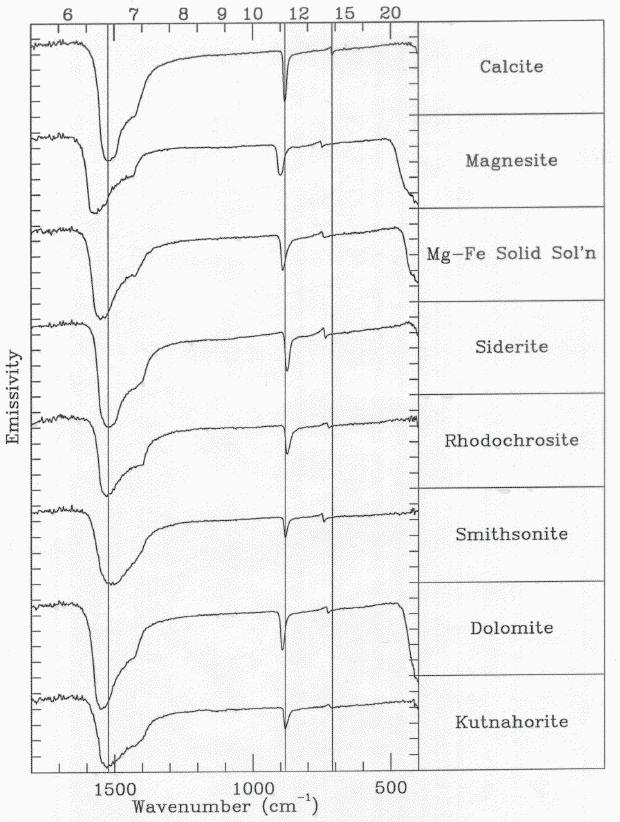
 Emissivity Spectra of Various Anhydrous Carbonates
Emissivity Spectra of Various Anhydrous Carbonates
The absorption features result from vibrations of the C-O bonds
in the carbonate anion. The subtle shifts in absorption band positions
arise from the slight variations in the crystal lattice between each
type of carbonate. These variations result from a different cation being
associated with each carbonate (e.g., Ca in calcite, Mg in magnesite).
Vertical lines are placed through the emissivity minima of calcite
for comparison to the other carbonate absorption band positions.
This figure is from Lane, M.D. and P.R. Christensen,Thermal
infrared emission spectroscopy of anhydrous carbonates, J. Geophys. Res., 102, 25581-25592, 1997.
 Go to the
Oxide Page
Go to the
Oxide Page
 Go back to my
Home Page
Go back to my
Home Page


